THE BIG SHOT
Oxford, Africa and the R21 malaria vaccine

Professor Ally Olotu had a problem. He was running the Tanzanian arm of a massive trial on a game-changing malaria vaccine, one part of an effort that spans four continents – but another disease, COVID-19, was threatening to stop the trial in its tracks. Travel was shutting down across the world and countries went into lockdown, while medical personnel were swamped treating the new disease – at a risk to their own lives. Just as the need for vaccines was hurled into sharp relief, progress on this vaccine against an ancient killer risked stalling altogether.
Yet, three years later, Olotu’s team, part of a consortium led by Professor Adrian Hill in Oxford, have announced to the world that the first highly effective vaccine against malaria was ready for use. In the face of everything from sceptical populations to vast logistical headaches, over countless all-hours video calls, the decentralised team would monitor and care for thousands of participants, and deliver vital proof that the R21-Matrix M vaccine was our most powerful weapon so far in the journey to eradicating malaria.
The story begins at least 30 years earlier, with patient and impossible-seeming efforts to develop vaccines against the parasite from Hill’s team in Oxford. Malaria has been a scourge for millennia, changing the course of empires and causing devastation to families around the world. The tiny parasite causes nearly 620,000 deaths each year, and most of its victims are children under five in sub-Saharan Africa.
The University of Oxford aims to change this with its ground-breaking R21 malaria vaccine. The vaccine is designed to prevent the disease, one of the deadliest infectious diseases in the world. This vaccine, the product of decades of global collaborations, has the potential to save millions of lives worldwide.
What we desperately need are new tools to improve malaria control, and this is the first vaccine that can be deployed at scale, that will be affordable, and can be used widely in Africa on a scale of hundreds of millions of doses each year.

Still a killer
We’ve made huge progress controlling malaria. But malaria still infects hundreds of millions – and its last hideouts will be hardest to clear


The world united against malaria : 1962 Ethiopian stamp. Wellcome Collection.
The world united against malaria : 1962 Ethiopian stamp. Wellcome Collection.
Malaria is caused by a parasite that is transmitted to humans through the bites of infected mosquitoes. The parasite multiplies in the liver and then spreads to infect red blood cells, leading to symptoms such as fever, chills and flu-like illness. If left untreated, malaria can lead to severe complications and death.
Malaria’s mark on history is clear. It has affected humans for many thousands of years. It was present in ancient Egypt, spread through the Roman Empire and affected India and China. It travelled to California in the Gold Rush and plagued solders in WWII. It affected Oliver Cromwell and Abraham Lincoln and, at least in a sense, has given us the gin and tonic.
Malaria’s modern impact has been most widespread in Africa. Global cases of malaria have dropped over the 20th century, and Africa now accounts for up to 95% of cases each year, and 96% of deaths. Although treatments and prevention methods, from antimalarial drugs to treated mosquito nets, have improved over the previous decades, the toll is still high. A vaccine would provide a crucial solution to save lives.

Malaria: a shape-shifting foe
Bacteria and viruses were tough targets for a vaccine. The parasite that causes malaria is a vastly more complicated enemy.

Oxford’s malaria vaccine, known as R21/Matrix-M, was developed by a team of researchers led by Professor Adrian Hill at the Nuffield Department of Medicine’s Jenner Institute. It is an erythrocytic vaccine, meaning it works by targeting the parasite on its way to and before it leaves the liver, preventing it from multiplying and causing illness.
Professor Hill’s hunt for a vaccine began nearly 30 years ago. He established the Jenner Institute in 2005; since then, his team has worked to identify the most effective way to stimulate an immune response against the parasite. They tested a variety of vaccine candidates before settling on the R21/Matrix-M vaccine.
One of the main challenges in developing a malaria vaccine is the complexity of the parasite itself. The parasite has a multi-stage life cycle, and it is constantly evolving, making it difficult to develop a vaccine that can provide long-lasting protection. Over time, the parasite has developed mechanisms to evade our immune system, making it even more challenging to develop an effective vaccine.

Malaria infection lifecycle
The parasite that causes Plasmodium falciparum malaria changes its form several times during its life cycle making it a hard target to hit.
Infection
- Sporozoite parasites, found in the saliva of infected female mosquitos, enter the blood after the mosquito bites a human to feed
Liver stage
- Sporozoites migrate to the liver, where a small number manage to invade liver cells (hepatocyte)
- After 7 days in the liver, the parasites enter the bloodstream as distinctively different merozoite parasite
- The liver stage of infection is asymptomatic. Individuals do not know they are infected and don’t get sick at this stage
Blood stage
- Merozoites attach to and enter red blood cells
- Merozoites replicate every two days, rupturing red blood cells and releasing more merozoite parasites
- This eventually causes symptomatic malaria infection, typically characterised by fever, chills and exhaustion – and in serious cases, anaemia and death
Transmission
- After a while the parasite forms sexual stage parasites known as gametocytes
- These migrate to blood capillaries under the skin, where they can be taken up by another feeding mosquito
- In the mosquito gut these sexual gametocytes mature into new sporozoites which migrate to the salivary glands of the mosquito to start the life cycle once again
The R21 vaccine works by targeting the sporozoite stage of the malaria parasite life cycle – just after the parasite enters the body and before it has time to spread through the liver and then into the blood stream. The vaccine generates an immune response that stops the parasite from developing and spreading. It contains Novavax’s Matrix-M adjuvant, which enhances immune system response and makes it more potent and more durable.

R21 vaccine: aiming for a bottleneck
The R21 vaccine hits the parasite when it has just arrived in the body, catching it before it grows out of control
Vaccination
- Three initial doses of vaccine, plus one a year later is given to children aged 5-36 months
- The vaccine targets protein antigens found on the surface of the sporozoite parasite
- The vaccine induces both protective antibodies (produced by B cells) and T cells
Infection
- As they move to the liver, the sporozoite parasites are intercepted by antibodies which prevent entry into the liver
Liver
- Sporozoite parasites that do make it to the liver and into liver cells are targeted by T cells
- The vaccine aims to prevent the development of the parasite’s next stage – the merozoite stage
To overcome the complex challenges presented by malaria, the researchers at the Jenner Institute worked with partners around the world – including the Serum Institute of India, the world's largest vaccine manufacturer and a key collaborator in ensuring hundreds of millions of doses of the Oxford-AstraZeneca COVID-19 vaccine could be manufactured and distributed all over the world. The partnership was essential in helping the researchers conduct clinical trials in areas the vaccine is needed most, such as sub-Saharan Africa where malaria occurs regularly.
The vaccine has undergone clinical trials in the UK, Thailand and several African countries, including a Phase III trial in Burkina Faso, Kenya, Mali and Tanzania that has enrolled 4,800 children since 2021. These trials have tested dosage, booster effectiveness and how the vaccine works against different seasonal patterns of malaria.
We have already seen the impact of the vaccine on our child, he has better health to succeed at school. Mostly, it is malaria that tires our population, especially children. I think the vaccine will help a lot of children to concentrate better in classrooms and they will have better chance to succeed.
The research was funded by a variety of sources, including the UK government, the Bill and Melinda Gates Foundation and the European Commission. The funding was essential in helping the researchers conduct clinical trials and develop the vaccine, which required significant resources and expertise.

Saving lives at scale
As the COVID-19 pandemic showed, vaccines only work if they reach tens of millions of people. But Oxford and its global partners have a plan.

The vaccine has shown promising results throughout its clinical trials. After successful early phase trials, the vaccine’s Phase III trials achieved high efficacy in infants and young children.
Countries in Africa have already started to approve the vaccine, with Ghana, Nigeria and Burkina Faso in 2023 so far; in October 2023, the R21 vaccine received World Health Organization recommendation for use in countries around the world.
Ensuring the vaccine works is only the first step in the road to getting into the arms of the people who need it. Oxford’s longstanding commercial relationship with the Serum Institute of India will play a crucial role in ensuring production at scale – and at modest cost, a crucial factor in ensuring the vaccine can be distributed widely. The Serum Institute has already established production capacity for 100 million doses per year, which will be scaled up to 200 million doses annually, meaning the vaccine could soon begin reaching children in Africa and around the world.

Future eradication: a combined effort
To get to a malaria free future we need to target each stage of the shapeshifting parasite independently
1. Liver stage vaccine
- Generates antibodies that correlate with protection against malaria
- Induces T cells which also contribute to protection in liver
- Low number of parasites to target
- If any merozoite parasites manage to develop, the life cycle will continue unhindered
KEY - Sporozoites: crescent-shaped parasite specialised for initial infection of the liver
2. Blood stage vaccine
- Generates antibodies that target antigens involved in red blood cell invasion
- T cells cannot target red blood cells so have little use
- Requires very high levels of antibodies to target larger number of parasites found at this stage
- A successful vaccine would prevent disease progression entirely
- Partially effective vaccines would reduce symptomatic disease
KEY - Merozoites: pear-shaped parasite specialised for entering red blood cells, where they mature and replicate, ready to infect further cells

3. Transmission blocking vaccines
- Transmission blocking vaccines do not offer any protection to the person receiving the vaccine
- Success depends on antibodies, taken up by mosquitos during feedings, targeting the parasite in the gut of the mosquito
- This prevents the parasite’s development, making the mosquito non-infectious
KEY - Sexual gametocytes: parasite equivalent of sperm and eggs
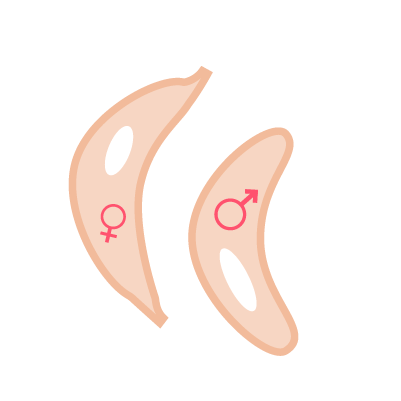
The University of Oxford's malaria vaccine research is a vital project that has the potential to save millions of lives and significantly reduce the burden of malaria in sub-Saharan Africa. Malaria is a challenging foe, but collaboration with partners around the world and funding from various sources has enabled the researchers to develop a vaccine with impressive results – one more tool in the fight to save lives.
With new vaccine types coming along … you have the real possibility that we could actually eradicate this parasite. That is the final goal.
As the vaccine is rolled out across the globe, it could pave the way for the development of future vaccines and the treatment of other infectious diseases, demonstrating the power of science and collaboration in improving global health outcomes.

Oxford and malaria: the road to eradication
One vaccine – even one this good – won’t end malaria on its own. Oxford, and researchers around the globe, are working on more.

This vaccine is part of Oxford’s wider work to understand, treat and prevent malaria. Find out more about Oxford’s research:
