Brain and mental health innovation at Oxford

Turning the tide on mental health
Mental Health and Dementia are a global healthcare crisis, affecting over one billion people worldwide.
Of those, over 400 million people are on the Alzheimer's disease continuum, with one person diagnosed every three seconds, and the figure expected to rise as the global population of those over 60 is set to double by 2050.
The global research community is trying to increase the availability of effective therapeutic and non-therapeutic options through service improvements, next-generation products, and the safe use of existing drugs for multiple conditions.
However, research spending on mental health remains low, compounded by expensive and complex clinical trials. While a large proportion of Alzheimer’s drugs have failed in the past, there seems to be light at the end of the tunnel as two amyloid monoclonal antibodies have been approved recently Aduhelm (Biogen) in 2022 and Leqembi (Eisai) earlier this year.
With its breadth and depth of research into conditions affecting the brain, ranging from anxiety and depression through to stroke and neurodegenerative diseases, Oxford University and its spinout companies are turning the tide on mental health.
Innovative spinouts are developing tools diagnostics to rapidly diagnose and identify conditions before they appear, creating new and innovative therapies across the spectrum of conditions affecting the brain, and building the infrastructure to accelerate research, drug discovery and clinical trials.
Oxford Brain Diagnostics

Rethinking brain health
One of the biggest hurdles in treating Alzheimer’s is diagnosis.
At present, diagnosis normally takes place after symptoms have begun to present themselves, shortening the window by which treatments – both pre-existing and potential – could have a positive impact on patient outcomes. Oxford Brain Diagnostics (OBD) is looking to change this.
Part of a family of imaging diagnostics firms to have emerged from the University, OBD is developing innovative breakthrough technologies to diagnose dementia and other brain-related neurodegenerative disorders, utilising diffusion MRI scans.
2019
Oxford Brain Diagnostics founded
The company was founded in 2019 by Dr Steven Chance, former Associate Professor in Clinical Neurosciences and now OBD’s CEO, and Mark Jenkinson, Professor of Neuroimaging and a global authority in MRI imaging software.
The duo combined their world-class expertise to develop Cortical Disarray Measurement (CDM®) technology, a software device that analyses grey matter quality, allowing insights into brain health at the cellular level.
CDM has been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA), allowing for a more accurate assessment of brain health based on changes at the cellular level, which was previously only possible through post-mortem analysis. The platform can also be applied across multiple neurological and psychiatric disorders, making it a valuable tool for personalised care for patients.
In the UK, the company is actively implementing on an NIHR grant awarded in 2020. This is a multi-centre project assessing CDM as a tool to diagnose mild cognitive impairment (MCI) due to Alzheimer’s disease and deliver positive health economic outcomes.
Internationally, OBD continues to expand its footprint into the US, Japan, and Asia. OBD has already been selected in a Phase 2 AD clinical trial as a secondary outcome measure with US based biotech, INmune Bio. In 2021/22, they completed a project in Parkinson’s with AbbVie which led to a publication at the International Congress of Parkinson’s Disease and Movement Disorders ® in Madrid.
OBD recently completed a clinical research pilot study with Niigata Hospital (NHO Dementia registry) in Japan and results of this collaboration will be made available in the forthcoming Alzheimer’s Association conference in Amsterdam in July of this year.
2023 will certainly be a busy year as the company is now moving ahead with submission for FDA 510K safety and efficacy clearance to launch CDM in the clinical sector.
Cytox
Defining genetic risk for dementia
Operating in the same space as Oxford Brain Diagnostics, Cytox is another Oxford spinout looking to identify those most at risk from Alzheimer’s before the disease manifests itself.
Alzheimer's disease affects over 46 million people globally, a figure that is projected to reach 131 million people by 2050, with an economic impact estimated to exceed $1tn per year in the next decade, according to the World Alzheimer's Report 2015.
There have been no new approved drug therapies for 10 years, and clinical trial failure rates are over 99%.
46 million
people are affected by Alzheimer's globally
Cytox's mission is to transform how new treatments are developed, people are screened, and patients are managed within the Alzheimer’s disease care pathway.
The company offers non-invasive, risk assessment, and patient stratification tools for Alzheimer's disease and dementia. Its genoSCORE tests assess an individual's genetic risk of cognitive decline due to Alzheimer's disease and only require a blood or saliva sample, enabling elderly and vulnerable patients to provide a sample from home.
The company was founded in 2006 by Dr Zsuzsanna Nagy, now Senior Lecturer in the Neurotrauma Research Group at Birmingham.
Brainomix
AI-enabled precision
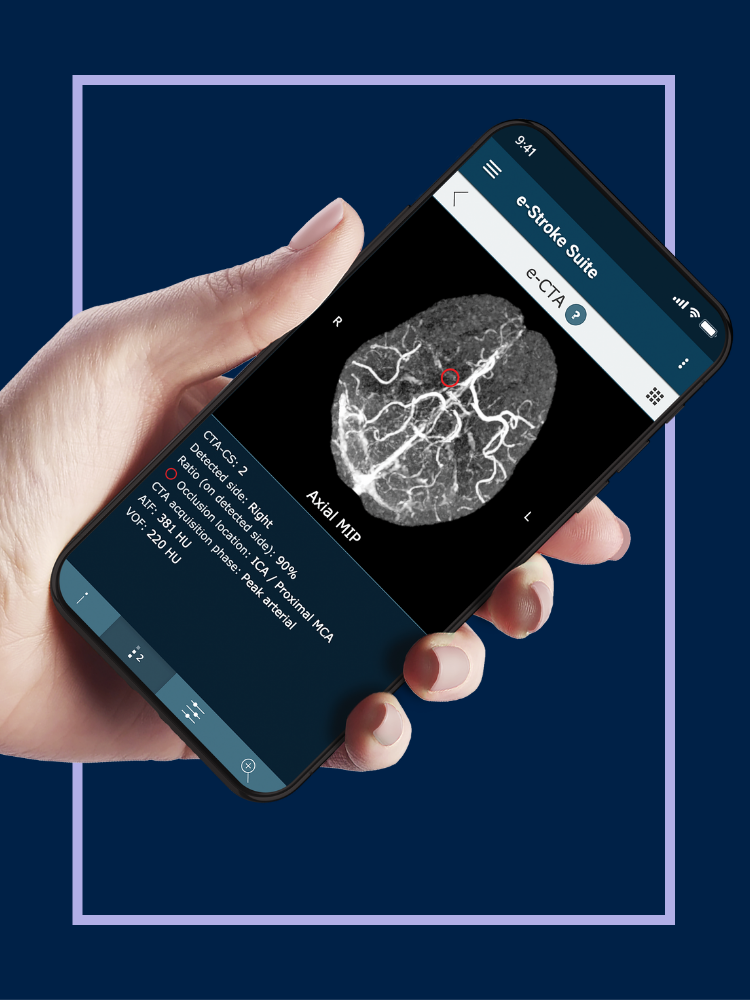
When it comes to the diagnosis of stroke, the seconds count. Clinicians need rapid and accurate assessment of the damage, which underpins the critical treatment decisions which need to be made at pace. Delivering that speed is what Brainomix was created for.
Led by CEO and co-founder Dr Michalis Papadakis, Brainomix has developed an imaging diagnostics platform that leverages artificial intelligence (AI) to improve the diagnosis and treatment of patients suffering from stroke.
Called e-Stroke, Brainomix’s platform analyses standard CT scans of the brain and uses AI algorithms to automatically detect and quantify the extent of stroke damage – providing clinicians with the rapid, in-depth picture they need to treat the condition.
The e-Stroke platform has been widely adopted by hospitals and stroke centres across Europe and Asia and has been shown to improve clinical outcomes for patients by reducing the time to treatment and increasing the accuracy of diagnosis.
Brainomix has also partnered with leading medical device companies to integrate e-Stroke into their imaging systems, making the technology more widely available to clinicians.
£16 million
in Series B funding
The company was founded in 2010 by Dr Papadakis while working as the Scientific Director of the preclinical stroke lab at the University, who sought to translate his research into practical solutions that could help sufferers of stroke.
His vision received support from Oxford's commercialisation arm, Oxford University Innovation, which helped to spin out the company and provide business support in its early days.
Brainomix has also attracted significant investment, including a £7 million Series A funding round co-led by Boehringer Ingelheim Venture Fund and Parkwalk Advisors in 2018, and a further £16m in Series B funding in 2021, also co-led by Boehringer and Parkwalk together with Tencent.
Brainomix's success in developing and commercialising AI-powered medical imaging technology demonstrates the potential of academic spinouts to translate innovative research into real-world impact that can benefit patients and healthcare providers.
As the company continues to expand, it is setting its sights towards stroke and onto lung conditions and cancer.
Ni2o
Thinking beyond the brain
With many candidates not clearing clinical trials, effective treatments for Alzheimer's and neurodegenerative diseases are proving a notoriously difficult problem to solve.
Yet, as the number of sufferers continue to mount - with stark predictions for the future as the global population grows and ages – a breakthrough is being sought by Ni2o using a tool perhaps somewhat unexpected in the field: Artificial Intelligence (AI).
The company, led by Dr Newton Howard, Professor of Computational Neuroscience and Functional Neurosurgery at the University, utilises AI to analyse and relieve symptoms for millions of people suffering from neurodegeneration and other mental health conditions.
The solution: the Kinetic Intelligent Wireless Implant (KIWI).
Neurodegenerative:
Resulting in or characterised by degeneration of the nervous system, especially the neurons in the brain
A small, self-contained, wirelessly connected, wirelessly charged chip, KIWI aims to replace current deep brain stimulation with AI. It records brain activity and delivers electrical and optical signals to target areas with algorithms that determine the therapeutic response and are continually improved by machine learning. Using this stimulation, Ni2o aims to restore cognitive ability to patients who suffer from debilitating mental conditions.
Existing deep brain stimulation devices are primitive and excessively invasive, requiring a lengthy procedure ending in permanent trepanation.
In contrast, KIWI is embedded via a minimally invasive operation. Neurons readily grow around the device’s carbon nanotube connectors, which deliver small electrical pulses that are the basis of the effectiveness of deep brain stimulation.
The Ni2o team believe this can help patients suffering from its first target: Parkinson’s Disease. Should KIWI prove a success with Parkinson’s, Ni2o will expand its focus to include Alzheimer’s disease.

Akrivia Health

Supporting the future of mental health
As society becomes more cognisant of the full range and scale of mental health conditions, the need to find rapid and effective therapies is clear.
However, with only 6% of UK health research spending going towards mental health, combined with the high cost of clinical trials, the research community must overcome several economic hurdles.
The team at Akrivia Health believe that with the right data and information, the global community can change the trajectory of research to develop better mental health and dementia drug treatments, pathways, and services.
Through combining patient data with artificial intelligence, the company is looking to accelerate research. Through its partnership with the National Health Service, Akrivia has access to 4.4 million de-identified patient records, 15 years of clinical interactions, 17 billion clinical data points, 900 million clinical notes and 5 million clinical attachments, which provides the most in-depth mental health and dementia dataset in the world.
The team apply AI to interpret this dataset, which results in insights that can accelerate drug discovery and optimise the expensive and lengthy clinical trials process.
17 billion
clinical data points
Founded in May 2019, Akrivia was spun out of Oxford University's Department of Psychiatry, with NHS Trusts as a partner.
The company's co-founders are Mike Denis, CEO, David Newton, COO, and Professor Sir Simon Lovestone. The three worked together on the UK's Clinical Record Interactive Search (CRIS) programme, the dataset which underpins Akrivia Health and enables health research across the UK.
Since its launch, Akrivia has expanded to work with academic and research institutions, healthcare organisations and industry, and is currently ramping up its international footprint. It is helping clients with drug discovery, patient recruitment and retention, clinical trial optimisation, and more.